 |
Click to enlarge |
Helium, Neon & Argon |
|
|
|
Introduction
The group of elements known as the rare, inert or noble gases possess
unique properties that make them important as geodynamic tracers. The
daughter isotopes fractionate readily from their parents, they are inert,
they give information about the degassing history of the mantle, the
formation of the atmosphere, and about mixing relationships between
different mantle components, and they differ from other geochemical
tracers in being gases and diffusing relatively rapidly.
The study of noble gases in basalts has led to the concept of a largely
undegassed, ancient, “primordial reservoir” in the lower
mantle. However, the best places on Earth to find the most “primordial”
material (i.e., high concentrations of noble gases, including
3He and 20Ne and high 3He/4He
and 20Ne/22Ne
ratios) are on mountain tops, in the stratosphere, and in deep-ocean
sediments. Evidence for recent additions of noble gases to the Earth
comes from deep sea sediments where high concentrations of 3He
(written [3He]), and high 3He/4He
are found (Merrihue,1964; Nier & Schlutter,
1990). However, mantle-derived basalts and xenoliths having similar “primordial”
or “solar” characteristics are generally attributed to
plumes from an undegassed primitive lower mantle reservoir. This is
based on the assumption that high 3He/4He and 36Ar/40Ar
ratios in basalts require high 3He and 36Ar
contents in their sources.
The critical issues are when and how the
“primordial” noble gases entered the Earth and whether
transport is always outwards. It is therefore important to understand
the noble gas budget of the Earth, both when it formed and as
it aged. The major part of terrestrial formation probably occurred
at high temperature from dry, volatile-poor, degassed particles.
Elements that formed the Earth during this time are known as “primordial”.
During the early stages of planetary formation, core differentiation
and massive bolide impacts, one of which was large enough to
form the moon, raised the Earth to temperatures that may have
been high enough for a substantial part of it to have melted,
and some to be vaporized. This is suggested by the fact that
a large fraction (30 to 58%) of the incompatible elements (e.g.,
U, Th, Ba, K, Rb) are in the Earth’s crust (Anderson,
1983; 1989), which is consistent with an extensively differentiated
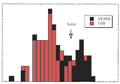
mantle. Strong degassing of the primordial volatiles occurred
during this period (Figure 1). The siderophiles have
very low abundances in crustal and mantle rocks (the bulk
silicate Earth) because they are in the core. |
Figure 1: Abundances of elements in the crust+mantle
relative to those in chondritic meteorites (from Anderson,
1989; Figure 8.2) |
The subsequent 4.55 billion years of planetary evolution was probably
more conducive to retention and accumulation of volatiles (except for
H and He, and possibly some Ne, which can escape from the atmosphere
to space), including the noble gases. Some of the components now in
the mantle fell to Earth in a late veneer, mostly around 3.8 Ga, the
period of late bombardment, that added material to the Earth after completion
of core formation (Kimura et al., 1974). This probably introduced
most of the volatiles and trace siderophiles (iron-lovers) that are
in the upper mantle today. However, what fraction of the noble gases
in mantle basalts is truly primordial vs. a late veneer – or even
later additions of cosmic dust – or recent contamination is unknown.
The hot-accretion model of Earth evolution contrasts with the “standard
model” of noble gas geochemistry that assumes that the bulk of
the mantle accreted in a primordial undegassed state with chondritic,
or cosmic, abundances of the noble gas elements. Such material is assumed
to make up the deep mantle today. This theoretical deep, isolated, undegassed
or little-degassed lower-mantle reservoir is considered to have high
[3He], [22Ne], [36Ar], 3He/4He
and 20Ne/22Ne, low 21Ne/22Ne
and to be tapped by plumes. The upper mantle, the presumed source of
mid-ocean ridge basalts (MORB), is considered to convect vigorously,
to be well-stirred, homogeneous and to have noble gas characteristics
the inverse of those of the lower mantle, listed above. Any high-3He/4He
or so-called “solar” neon (see below) found along mid-ocean
ridges is presumed to have been transported into the upper mantle by
plumes.
Isotopic Ratios
The most useful light noble gas isotopic ratios are shown in Table
1
Table 1: The most useful light
noble gas isotopic ratios. * signifies radiogenic or nucleogenic.
|
3He/4He* |
3He/22Ne |
3He/20Ne |
3He/36Ar |
4He*/21Ne* |
4He*/40Ar* |
|
|
20Ne/22Ne |
|
|
|
21Ne*/22Ne |
|
|
|
40Ar*/36Ar |
|
|
|
Basic data concerning the noble gases He, Ne, &
Ar are given in Table 2.
| Table
2: Noble gas isotopes, their sources and half-lives. |
| Isotope |
Source |
Half
life |
| 3He |
stable |
|
| 4He* |
decay
of U+Th. 238U=8(4He)+206Pb,
235U=7(4He)+207Pb,
232Th=6(4He)+208Pb |
103
– 104 Ma |
| 20Ne |
stable |
|
| 21Ne* |
nucleogenic
decay chains of 24Mg, 18O when irradiated
by U+Th decay products. Thus produced at the same rate as
4He |
|
| 22Ne |
some
is primordial and some produced in a similar way to 21Ne
but only in very small amounts |
|
| 36Ar |
stable |
|
| 37Ar* |
from
fission |
|
| 38Ar |
stable |
|
| 39Ar |
cosmic
rays |
|
| 40Ar* |
decay
of 40K & 40Ca. Comprises 99.6% of
the Ar in the atmosphere. |
103
Ma |
Helium
There are few constraints on how much helium may
be in the Earth or when it arrived. It is readily lost from the atmosphere
via escape into space. It is therefore not very useful for studying
the outgassing history of the Earth. Neon and argon are potentially
more useful in this regard since they do not escape. We know, for
example, that most of the argon in the Earth is now in the atmosphere,
even though much of it was produced over time and was not in the mantle
during the earliest high temperature phases of accretion and evolution.
This argues against there being a large undegassed reservoir in the
Earth.
|
Both melting and degassing fractionate the noble gases, and
separate the parent from the daughter isotopes. Helium and the
light noble gases are more readily retained in magma than the
heavy noble gases during degassing. Thus, the He/Ne and He/Ar
ratios of different basalt types contain information about their
degree of degassing. The He/Ne and He/Ar ratios of MORB are
generally higher than OIB (Figure 2) (Ozima & Podosek,
2002) suggesting that MORB is more degassed than OIB. Nevertheless,
[He] is higher in MORB than in OIB, which suggests that OIB
initially contained less [He] and possibly less [Ne] than MORB,
prior to MORB degassing. Some of the more noble-gas-rich igneous
rocks are the “popping rocks” found along the mid-Atlantic
ridge (e.g., Sarda & Graham, 1990; Javoy
& Pineau, 1991; Staudacher et al., 1989).
Ordinary MORB appear to be degassed versions of these popping
rocks, and even some of these gas-rich rocks appear to have
lost some gas. |
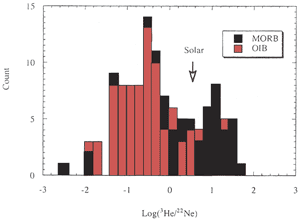
Figure 2: Histogram of 3He/22Ne
in MORB and OIB. “Solar” indicates the cosmic abundance
ratio. MORB and OIB are fractionated in opposite directions
relative to the solar ratio [from Ozima & Igarashi, 2000]. |
A plot of 4He/40Ar
vs [4He] and related inter-element plots
(e.g., 3He/21Ne vs. [He])
shows a continuous transition from MORB to OIB to
air (e.g., Figure 3). The MORB to OIB trends could
be interpreted as air-contamination of noble gases
degassed from MORB and trapped in vesicles or cumulates.
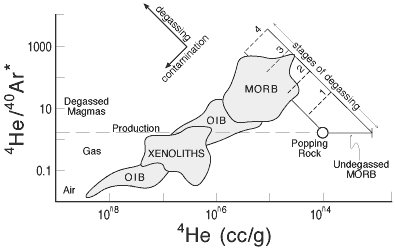
Figure 3: Plot showing relation between
helium and argon isotopes in MORB and OIB and the effects of
degassing and “contamination” with air and older
CO2-rich vesicles (Anderson, 2000). |
So-called primitive ratios of noble gas
isotopes may be preserved by ancient separation
of He and Ne from U+Th and storage of the
gas in low-U+Th environments such as depleted
lithosphere or olivine cumulates (Anderson,
1998a,
1998b;
Natland, 2003). In this way, the
radiogenic isotopes 4He and 21Ne
are not added to the gas and old isotopic
ratios can be “frozen in”. This
contrasts with the situation in more fertile
materials such as MORBs or undepleted peridotite,
where U+Th contents are relatively high
and continued radiogenic and nucleargenic
ingrowth occurs (see also Helium
Fundamentals page).
|
Neon
Neon isotope ratios for mantle rocks are
typically displayed on a “3-isotope
plot” of 20Ne/22Ne
vs. 21Ne/22Ne (Figure
4; see Graham, 2002). Neon isotope
ratios are more susceptible to atmospheric
contamination than helium isotope ratios
because Ne is more abundant in the atmosphere.
Most basalts plot on what appear to be mixing
lines between atmospheric values (~10),
and solar wind – SW or interplanetary
dust particle – IDP values (13.7)
of 20Ne/22Ne. The
corresponding values for the 21Ne/22Ne
ratios are 0.029 (air), > 0.04 (OIB)
and > 0.06 (MORB).
|
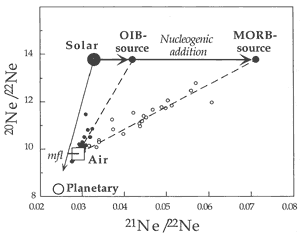
Figure 4: “Three-isotope plot”
of 20Ne/22Ne vs. 21Ne/22Ne. |
The mixing line for MORB extends to greater values
of 21Ne/22Ne than the mixing line for OIB, indicating
more 21Ne ingrowth in MORB than in OIB. This is analogous
to the higher 4He/3He ratios found in some MORB
samples compared to the extremes found in some OIB samples. The 20Ne/22Ne
ratios in mantle derived rocks, both MORB and OIB, extend from atmospheric
to the solar or IDP ratio (Anderson, 1993; Allegre et
al., 1993). The identification of solar-like (“primordial”
or IDP) Ne isotopic ratios in some OIB and MORB samples implies that
solar neon trapped within the Earth has remained virtually unchanged
over the past 4.5 Gyr (the standard “primordial mantle”
model) or, alternatively, that noble gases have been added to the
mantle or the samples more “recently” (i.e.,
since 2 Ga), perhaps from noble-gas-rich sediments. Some basalts and
some diamonds essentially have pure “solar” (SW or IDP)
20Ne/22Ne ratios. The fact that the 20Ne/22Ne
ratio of rocks or magmas thought to come from the mantle is greater
than the atmospheric ratio is a paradox for standard models of mantle
degassing and atmospheric evolution because 20Ne and 22Ne
are essentially primordial in the sense that 20Ne is stable
and only very small amounts of 22Ne are produced. Their
ratio is thus expected to be uniform throughout the Earth (Craig
& Lupton, 1976), if the solar system reservoir is uniform
and invariant with time.
Neon and Helium
The 3He/22Ne and 4He/21Ne
ratios observed in mantle xenoliths and basaltic glasses vary by orders
of magnitude and define a linear correlation with a slope of unity
which passes through the point defined by the mean primordial 3He/22Ne
ratio (= 7.7) and the radiogenic 4He to nucleogenic 21Ne*
production ratio (= 2.2 x 107) (Figure 5, see Dixon
et al., 2001). The linear correlation implies that the elemental
fractionation event (perhaps degassing of magma), which enriched MORB
glasses in [He] with respect to [Ne], is recent, otherwise in-growth
of radiogenic 4He and nucleogenic 21Ne would
have systematically shifted the data points from the correlation line.
Basalts exhibit positive correlations between 3He/22Ne
and [3He], and between 4He/21Ne*
and [4He]. The 3He/22Ne, 4He/21Ne*
and 4He/40Ar* ratios in MORB glasses are systematically
higher than the primordial 3He/22Ne ratio or
the radiogenic 4He*/nucleogenic 21Ne* and radiogenic
4He*/radiogenic 40Ar* production ratios.
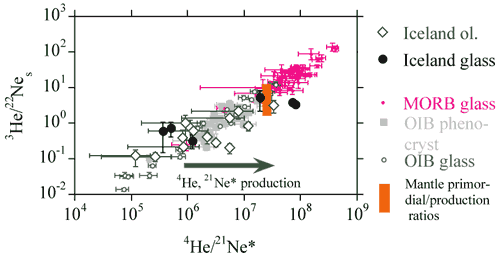
Figure 5: Plot of 3He/22Ne vs.
4He/21Ne (from, Dixon et al., 2001).
|
The production rate of 21Ne parallels
that of 4He since they both result from the decay of U+Th
(Table 2). The 3-isotope plot showing OIB (Loihi) as forming one trend
from atmosphere to solar and MORB as forming another (Figure 4) can
be interpreted as a rotation of the OIB trend by nucleogenic ingrowth,
i.e., MORB source is aged OIB source. In this simple model
MORB = OIB + time. OIB and MORB could have evolved from a common parent
at some time in the past. Old MORB gases (from ancient degassed MORB)
stored in a depleted refractory host (e.g., olivine crystals,
U+Th-poor lithosphere) will have high 3He/4He
and low He/Ne compared to current MORB magmas. One of the mantle “reservoirs”
for noble gases may be isolated gas-filled inclusions or vugs. This
“reservoir” would contribute nothing besides He and CO2
and perhaps some Ne, thereby decoupling He form other isotopic tracers.
Argon
The main isotopes of argon found on Earth are 40Ar,
36Ar, and 38Ar. Naturally occurring 40K
with a half-life of 1.250 x 109 years, decays to stable
40Ar.
Atmospheric He has a very low abundance due to its
complete escape from the atmosphere, and even atmospheric Ne is believed
to have been significantly depleted in the atmosphere by intense solar
irradiation. The Ne three-isotope plot allows atmospheric contamination
to be monitored for this isotope. On the other hand, the heavy rare
gases (argon, krypton and xenon) have accumulated in the atmosphere
over Earth history, and contamination is a serious problem in the
study of mantle values.
The values of 40Ar/36Ar (or 40Ar*/36Ar)
measured, or estimated, in various materials are:
air ~ 300
OIB atmospheric to ~ 13,000
MORB atmospheric to ~ 44,000
In the standard model, which assumes a lower-mantle
source for OIB, these high ratios for OIB and MORB, compared with
air, have been taken to indicate that both the upper (MORB) and the
lower mantles (OIB) have been degassed such that little 36Ar
remains, but large quantities of 40Ar have been produced
by radiogenic decay and are retained. The higher 40Ar/36Ar
in mantle rocks than in the atmosphere contrasts with the situation
for He, where 4He/3He is usually much higher
in the atmosphere than in either OIB or MORB as a result of the preferential
escape of the lighter 3He atom. Much of the He in the atmosphere
entered it in the last million years and is therefore dominated by
4He.
Low 40Ar/36Ar plays the same
role in the standard noble gas model as high 3He/4He
in that low radiogenic ratios are taken as proxies for less degassing
and more primitive reservoirs. Others have argued, however, that this
does not place strong constraints on the argon isotope ratio of the
“lower mantle undegassed reservoir” because plumes may
have been contaminated with radiogenic argon during ascent through
the MORB source.
The concept of a relatively less degassed mantle
reservoir with respect to heavy rare gases was contested by Fisher
(1983; 1985) who argued that low 40Ar/36Ar is
a result of atmospheric or seawater contamination. Seawater has 2
– 4 orders of magnitude more 36Ar than mantle basalts
and two orders of magnitude less 3He. Hence, Ar isotope
ratios in magmas can be significantly changed by seawater without
affecting their He signature. Measured [36Ar] covers the
same range for MORB and OIB (Ozima & Igarashi, 2000),
and [3He] is higher for MORB than for OIB. This also argues
against OIB arising from a reservoir much less degassed than the MORB
reservoir. This model for Ar is at odds with the standard model for
He that considers the lower mantle to be little degassed. The lower
ratios for OIB can thus be explained by more extreme atmospheric contamination,
or a potassium-poor- or younger source. Usually, however, unradiogenic
ratios are attributed to high abundances of the primordial isotope,
i.e., to undegassed or primordial reservoirs. The 20Ne/22Ne
ratios for mantle rocks that are significantly higher than atmospheric
indicate that the source of noble gas in both OIB and MORB cannot
be mainly present-day atmospheric.
40Ar is derived from 40K and
is retained by the atmosphere. The initial amount
of 40Ar contained in the Earth is negligible
compared with the amount that was produced subsequently
radiogenically. This makes it a useful tool with
which to study mantle degassing. The amount of K
in the crust and mantle may be estimated by calculating
the mix of mantle and crustal components that satisfies
the cosmic ratios of the refractory elements (Anderson,
1989). The amount of K in the silicate Earth (the
mantle and crust) so determined is 151 ppm of which
46% is in the crust. The amount of 40Ar
in the atmosphere represents 77% of that produced
by the decay of this amount of potassium over the
age of the Earth. This implies that either 23% of
the Earth is still undegassed or that the degassing
process is not 100% efficient i.e., that
there is a delay between the production of 40Ar
and its release into the atmosphere. Ozima
(1998) has suggested that Ar may be compatible at
depths > ~ 150 km and thus not be easily extracted
from the Earth by volcanism. A similar phenomenon
appears to occur with 4He, which does
not degas at a high enough rate to correspond to
the outflow of heat generated by U+Th decay. This
is known as the helium heat-flow paradox. Since
it is more difficult to degas the mantle now than
during the early, high-temperature period of Earth
history, it is likely that the stable isotopes –
those that have not accumulated over time –
are more degassed than the radiogenic and nucleogenic
ones e.g., 4He and 40Ar.
The “two reservoir”
model
The partially degassed or “two reservoir”
model for the mantle was originally proposed
to explain argon isotope systematics (Hart
et al., 1979), and was applied to helium
by Kaneoka & Takaoka (1980).
Kaneoka & Takaoka (1980) based
their case on rare gas compositions in phenocrysts
from Haleakala volcano, Hawaii; these were
subsequently shown to be contaminated with
atmospheric argon and cosmogenic helium
(Fisher, 1983; Kurz, 1986a).
Some ocean island basalts, most notably
from Hawaii, Iceland and the Galapagos have
elevated 3He/4He values
compared to most MORB samples. Hence the
two-reservoir model for mantle helium was
resurrected and is now widely accepted.
|
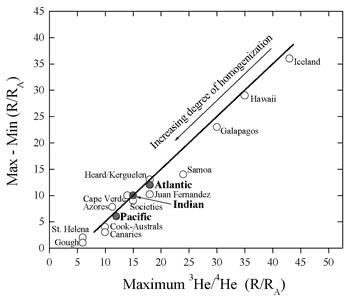
Figure 6: Maximum value of R vs. the spread
in values showing that high-R islands also have the largest
range in values (figure provided by A. Meibom).
|
An alternative way of looking at the helium data
is via histograms. Both OIB and MORB samples define
smooth distributions, with the MORB distribution
being more Gaussian-like and with smaller variance.
The so-called “high-He” hotspots exhibit
very large variance (Figure 6). This is suggestive
of the predictions of the central limit theorem
and implies that mid-ocean ridges sample larger
volumes of a heterogeneous mantle than do oceanic
islands (Anderson, 2000).
Another test of the two-reservoir
model was made by comparing helium and heat fluxes
(O’Nions & Oxburgh, 1983). These
fluxes must be related because the decay of uranium
and thorium produces both radiogenic helium (alpha
particles) and heat. O’Nions & Oxburgh
(1983) calculated the concentration of U necessary
to generate the observed helium and heat fluxes,
assuming an instantaneous steady-state. The results
were surprising. The amount of uranium required
to generate 88% of the Earth’s oceanic helium
flux only produces 3% of the oceanic heat flow.
O’Nions & Oxburgh (1983) proposed
that a boundary layer inhibits upward transport
of helium from the “primordial” reservoir
(assumed to be very deep) much more effectively
than the transport of heat. They envisaged this
boundary layer near 650 km depth, at the phase-change
boundary separating the upper and lower mantle.
This implies that the whole lower mantle is a kind
of primordial helium reservoir.
The other alternative is that helium, along with
CO2, from degassing magmas, is trapped in the shallow mantle
which, along with U+Th, contributes to the low 3He/4He
of some upper mantle components, and the ubiquitous carbonatitic metasomatism.
O’Nions & Oxburgh (1983) ignored secular cooling,
the diversion of heat from continental regions toward the thin-lithosphere
oceanic regions, and the delay of heat reaching the surface. Nevertheless,
their results suggest that helium is not as mobile an element as generally
thought, opening up the possibility that helium in various upper mantle
components with differing He/U ratios, can diverge in situ
in their isotopic ratios, removing the requirement for ancient or
large gas-rich sources (see also Helium
Fundamentals page)
A challenge for the two-reservoir
model is to explain the respective concentrations
of helium and other rare gases in the reservoirs.
If OIBs come from an undegassed source, they would
be expected to contain more helium than MORB glasses
from the degassed upper mantle. However, OIB glasses
typically have ten times less 3He than
MORB (Fisher, 1985). This has been explained
as degassing, but that cannot explain the higher
He/Ne in MORB than in OIB. This observation has
sometimes been called one of the Helium Paradoxes,
and there are several (Anderson, 1998a,
1998b).
The dynamics of mantle convection and melt segregation
under ridges must be different from under oceanic
islands and seamounts. Ridge magmas probably collect
helium from a greater volume of mantle and blend
magmas from various depths, during the melting and
eruption process. This would even out the extremes
in MORB basalts. Most workers, however, favor the
two-reservoir model for the noble gases, although
the evidence is far from definitive, and the assumption
of an undegassed lower mantle causes more problems
that it solves.
Reservoirs, components and recycling
The concepts of primordial and homogeneous degassed
reservoirs in the Earth were challenged by Anderson (1993)
and Meibom et al. (2003) who argue that there are mantle
components with a large range of helium isotopic ratios – because
of the distribution in ages and 3He/U ratios – rather
than two reservoirs with distinctive compositions. The high “solar”
Neon component has several possible sources including IDP and “old”
gas trapped in depleted U+Th rocks or minerals.
“Primitive” signatures have been attributed
to the subduction of IDP (Allegre et al., 1993; Anderson,
1993). These particles accumulate in ocean-floor sediments (Merrihue,
1964). Cosmic dust has very high 3He/4He ratios
and [3He] and can fall to Earth without burning up in the
atmosphere (Nier & Schlutter, 1990). In this way ocean-floor
sediments develop a “primordial” helium isotope signature.
This would have been even more true in the past if ET flux was higher
then, and oceanic organic productivity lower.
The rare gases in cosmic dust particles are encapsulated
in silicate and magnetite grains – the magnetite grains are
easier to collect from the seafloor and have been most studied –
which are relatively resistant to thermal degassing (Matsuda et
al., 1990). Therefore, the cosmic helium in ocean-floor sediments
might survive the subduction process and be transported into the shallow
mantle, along with other “fragile” and “mobile”
materials such as water and 10Be. In contrast, atmospheric
rare gases trapped in ocean-floor sediments are very susceptible to
thermal degassing.
Arguments against subduction of “primordial”
noble gas components of cosmic and ET origin into the mantle include:
1. rapid diffusive loss from individual grains,
2. the low average 3He/4He ratio in current
MORB and island arc basalts,
3. the low current ET influx of 3He, and
4. the large current rate of mantle 3He loss.
Diffusive loss of a component from a rock or sediment
does not mean it is completely lost from the system. It could also
become trapped in other host components in the upper mantle at subduction
zones. The low average 3He/4He ratio of MORB
can be explained by large volume averaging of a heterogeneous mantle
with a wide range of 3He/4He values (Meibom
& Anderson, 2003). 3. and 4. are uniformitarianism arguments,
i.e., the current ET and mantle fluxes are assumed to have
remained constant throughout Earth history and ancient sediments are
assumed to have had the same composition as modern sediments. Many
basalts with so-called primitive or primordial noble gas signatures
show, additionally, evidence for atmospheric contamination, e.g.,
40Ar/36Ar in some OIB is similar to the atmospheric
value. This combination of surface characteristics with ones conventionally
attributed to the deep mantle is a paradox in the standard model for
noble gases.
If cosmic dust and oceanic sediments
survive the “subduction barrier” against
atmospheric rare gases, they have the potential
to deliver He with a primordial signature into the
mantle, probably its shallow levels (Allegre
et al., 1993, Anderson, 1993). The
3He in OIB is a small fraction of the
total 3He degassed from the mantle and
in volume is comparable to the small amount of 3He
brought onto the seafloor by IDP. However, much
of the 3He in the mantle may have come
in as part of the late veneer; it is subduction,
or the Archean equivalent, at some 1 – 2 Ga
that is relevant, not today’s flux (Anderson,
1993). Allegre et al. (1993) noted that
the 3He/20Ne ratio in cosmic
dust is one to two orders of magnitude lower than
3He/20Ne in the upper mantle.
Helium has a much greater diffusivity than Ne, which
would promote its preferential degassing from cosmic
dust grains during subduction (Hiyagon,
1994) although this does not mean that it immediately
escapes the mantle. It appears that subduction of
cosmic dust having the properties of today’s
dust cannot contribute more than a small fraction
of the mantle 3He budget without causing
excessive enrichment of 20Ne in submarine
glasses. The preferred explanation for the high
3He/4He components in both
OIB and MORB mantle is, therefore, evolution in
low-U+Th environments such as olivine cumulates
(Natland, 2003) or depleted lithosphere
(Anderson, 1998a,
1998b).
While Loihi, Iceland, Yellowstone and the Galapagos have the highest
3He/4He components – usually called the
most primordial He compositions – some ocean islands such as
Tristan, Gough, islands in the SW Pacific and the Azores, and some
components in Yellowstone, Iceland and the Galapagos, have 3He/4He
ratios lower (more radiogenic) than MORB. These low 3He/4He
ratios require a component of radiogenic He from a long-lived U+Th-rich
source such as recycled oceanic crust or sediments in the mantle,
as inferred from lithophile isotope data. Parallel recycling of refractory
peridotite or olivine-rich cumulates could then be responsible for
high 3He/4He ratios and these components will
not have enriched signatures for other isotopes. None of these components
need to be recycled very deep into the mantle.
Some of the arguments against recycling involve steady-state or uniformitarian
principles such as constancy of ET input, volcanic output and sedimentary
and IDP compositions. Kellogg & Wasserburg (1990) assumed
a steady state between supply and degassing in order to determine
the residence time of He in the upper mantle. They argued that ridges
are the principal sites of helium escape from the upper mantle (whereas
hot spots would dominate in outgassing a deep, large, gas-rich reservoir
in “the lower mantle”).
Another approach to this problem is to dismantle
the two reservoir model entirely. Anderson (1989), Coltice
& Ricard (1999), Anderson (2001), Meibom &
Anderson (2003) and Seta et al. (2001) suggested that
a “primordial” or “relatively undegassed”
reservoir does not exist. Instead, they argued that all the mantle
is degassed, but some parts (e.g., the MORB “reservoir”)
are more enriched in radiogenic helium due to high-U+Th concentrations
combined with great age. Anderson (2000) and Meibom &
Anderson (2003) suggested that OIB are derived from a heterogeneous
shallow mantle through sampling that is different from that which
produces MORB. In contrast, other workers assume a lower-mantle plume
source with very high 3He/4He and [3He],
which must be isolated and preserved against convective homogenisation
with the more radiogenic upper mantle.
The latter model has several problems. Mantle convection
could allow large lumps of the lower mantle to be preserved intact,
without being homogenised (van Keken & Ballentine, 1998;
1999) but realistic models cannot preserve lower-mantle domains large
enough to preserve a significant primordial helium reservoir. On the
other hand it is difficult to convectively homogenize the upper mantle
(Meibom & Anderson, 2003). Extremely high 3He/4He
data from Baffin Bay (3He/4He = 49.5 R/RA)
and Iceland (3He/4He = 42.2 R/RA)
require that the hypothetical ancient undegassed reservoir must be
even more gas-rich than previously thought (Stuart et al.,
2003; Foulger & Pearson, 2001). Higher 3He/4He
and solar Ne ratios, in the standard model, require even less degassing.
In alternate models, these components are viewed as having been isolated
in [He]-poor, U+Th-poor environments rather than in [He]-rich ones.
Finally, low 3He/4He values
observed at some hotspots may result from shallow mixing with radiogenic
sources. The average 3He/4He value of the undegassed
source might need to be even higher than proposed. In the recycling,
heterogeneous model both the high- and low-3He/4He
components arise from recycling, but the extreme values – high
and low – are averaged out at ridges by large-volume mantle
sampling. Primordial He may thus be present in the mantle but a large,
coherent, ancient, primordial, undegassed region is unlikely.
Solar neon in the Earth
Evidence for non-atmospheric neon in the mantle
was presented by Craig & Lupton (1976) on samples of
MORB and volcanic gases. These are enriched in 20Ne, as
well as nucleogenic 21Ne, relative to 22Ne.
These 20Ne-enriched components were attributed to primordial
components in the Earth, possibly solar neon. Elevated 20Ne
abundances were also found in diamonds (Honda et al., 1987;
Ozima & Zashu, 1988; 1991). Diamonds represent in
situ solid samples of the mantle. Therefore, Ozima &
Zashu (1988; 1991) suggested that diamonds sample a solar neon
reservoir in the Earth, whereas the present-day atmosphere has been
depleted in 20Ne by mass fractionation. They argued that
bombardment of the early Earth by radiation caused massive blow-off
of a primitive solar-type atmosphere, leaving a residue enriched in
heavy neon. Most MORB samples can be explained by three-component
mixing of solar-type, atmosphere-type and nucleogenic neon (Figure
4).
Basalt glasses from Loihi and Kilauea reveal a range
of Ne isotope ratios, stretching from atmospheric to 20Ne-enriched
compositions (Honda et al., 1991; Hiyagon et al.,
1992). The enriched end of this array approaches the solar wind composition.
Honda et al. (1991) and Hiyagon et al. (1992) attributed
all Ne in the Earth’s interior to mixing between solar and nucleogenic
isotopes. The arrays of MORB and OIB were attributed to variable atmospheric
contamination of this solar + radiogenic mantle Ne. Loihi Ne samples
were attributed almost entirely to atmospheric contamination.
Allegre et al. (1993) attributed these signatures
to the subduction of cosmic dust particles accumulated in deep-sea
sediments. These dust particles become impregnated with Ne from the
solar wind during their exposure in space. Analysis of this material
in the atmosphere and in deep sea sediment reveals 20Ne/22Ne
ratios which span the range between atmospheric and solar compositions
(e.g., Nier & Schlutter, 1990). Matsuda
et al. (1990) suggested that these particles could survive the
“noble gas subduction barrier” and deliver cosmic (solar)
Ne to the deep mantle. This could explain the high 20Ne/22Ne
ratios of submarine glasses without having to invoke primordial Ne
in the Earth. Experimental studies by Hiyagon (1994) suggested
that Ne would be completely extracted from cosmic dust (the magnetic
portion) in a partial vacuum within 3 years at 500°C. But this
assumes that no other host in the sediments or mantle can take up
the gases, and that the non-magnetic fractions of cosmic dust are
not more retentive. Evidence from Iceland (Dixon et al.,
2000; Moreira et al., 2001) implies that some basalts, as
well as oceanic sediments, contain essentially pure solar or IDP neon
components, termed “primordial” by the authors.
|
- Allegre, C. J., Sarda, P. and Staudacher, T. (1993).
Speculations about the cosmic origin of He and Ne
in the interior of the Earth. Earth Planet. Sci.
Lett. 117, 229-233.
-
Anderson, Don L., (1983). Chemical
composition of the mantle,J. Geophys. Res.,
88 supplement, B41-B52.
-
Anderson, D. L. (1993). Helium-3
from the mantle: primordial signal or cosmic dust?
Science 261, 170-176.
-
-
-
Anderson, Don L., (1989), Theory
of the Earth, Blackwell Scientific Publications,
Boston, 366 pp.
-
Anderson, Don L., (2000), The
statistics of helium isotopes along the global spreading
ridge system and the Central Limit Theorem, Geophys.
Res. Lett., 27, 2401-2404.
-
Anderson, D. L. (2001). A statistical
test of the two reservoir model for helium isotopes.
Earth Planet. Sci. Lett. 193,
77–82.
-
Coltice, N. and Ricard, Y. (1999).
Geochemical observations and one layer mantleconvection.
Earth Planet. Sci. Lett. 174,
125–37.
-
Craig, H. and Lupton, J. E.
(1976). Primordial neon, helium, and hydrogen in
oceanic basalts. Earth Planet. Sci. Lett.
31, 369-385.
-
Dixon, E. T., Honda, M., McDougall,
I., Campbell, I. H. and Sigurdsson, I. (2000). Preservation
of near-solar neon isotopic ratios in Icelandic
basalts. Earth Planet. Sci. Lett. 180,
309-324.
-
Dixon, E.T., Honda, M. and McDougall,
I., (2001). The origin of noble gas isotopic heterogeneity
in Icelandic basalts, 11th Annual Goldschmidt Conference.
-
Fisher, D. E. (1983). Rare gases
from the undepleted mantle? Nature 305,
298-300.
-
Fisher, D. E. (1985). Noble
gases from oceanic island basalts do not require
an undepleted mantle source. Nature 316,
716-718.
-
Foulger, G.R. and D.G. Pearson
(2001). Is Iceland underlain by a plume in the lower
mantle? Seismology and helium isotopes, Geophys.
J. Int. 145, F1-F5.
-
Graham, D. W., Noble gas isotope
geochemistry of mid-ocean ridge and ocean island
basalts; characterization of mantle source
reservoirs. In: Noble Gases in Geochemistry
and Cosmochemistry, Eds. D. Porcelli, R. Wieler
& C. Ballentine, Reviews in Mineralogy and Geochemistry,
Mineral. Soc. Amer., Washington, D.C., pp. 247-318,
2002.
-
Hart, R, Dymond, J. and Hogan,
L. (1979). Preferential formation of the atmosphere-sialic
crust system from the upper mantle. Nature
278, 156-159.
-
Hiyagon, H. (1994). Retention
of solar helium and neon in IDPs in deep sea sediment.
Science 263, 1257-1259.
-
Hiyagon, H., Ozima, M., Marty,
B., Zashu, S. and Sakai, H. (1992). Noble gases
in submarine glasses from mid-ocean ridges and Loihi
seamount: constraints on the early history of the
Earth. Geochim. Cosmochim. Acta 56,
1301-1316.
-
Honda, M., Reynolds, J. H., Roedder,
E. and Epstein, S. (1987). Noble gases in diamonds:
occurrences of solar-like helium and neon. J.
Geophys. Res. 92, 12,507-12,521.
-
Honda, M., McDougall, I., Patterson,
D. B., Doulgeris, A. and Clague, D. A. (1991). Possible
solar noble-gas component in Hawaiian basalts. Nature
349, 149-151.
-
Javoy, M. and F. Pineau, (1991).
The volatiles record of a “popping”
rock from the mid-Atlantic ridge at 14°N: Chemical
and isotopic composition of gas trapped in the vesicles,
Earth Planet. Sci. Lett. 107,
598-611.
-
Kaneoka, I. and Takaoka, N. (1980).
Rare gas isotopes in Hawaiian ultramafic nodules
and volcanic rocks: constraints on genetic relationships.
Science 208, 1366–8.
-
Kellog, L. H. and Wasserburg,
G. J. (1990). The role of plumes in mantle helium
fluxes. Earth Planet. Sci. Lett. 99,
276–89.
-
Kurz, M. D. (1986a). Cosmogenic
helium in a terrestrial rock. Nature 320,
435-439.
-
Matsuda, J., Murota, M. and Nagao,
K. (1990). He and Ne isotopic studies on the extraterrestrial
material in deep-sea sediments. J. Geophys.
Res. 95, 7111-7117.
-
McDougall, I. and Honda, M.
(1998). Primordial solar noble gas component in
the Earth, in The Earth's Mantle, I.N.S.
Jackson, Ed., Cambridge University Press, Cambridge,
159-87.
-
Meibom, A., D.L. Anderson, N.H.
Sleep, R. Frei, C.P. Chamberlain, M.T. Hren and
J.L. Wooden, (2003). Are high 3He/4He
ratios in oceanic basalts an indicator of deep-mantle
plume components?, Earth Planet. Sci. Lett.
208, 197-204.
-
Meibom, A. and D.L. Anderson,
(2003). Statistical upper mantle assemblage, Earth
Planet. Sci. Lett. 217, 123-139.
-
Merrihue, C. (1964). Rare gas
evidence for cosmogenic dust in modern Pacific red
clay. Ann. N. Y. Acad. Sci. 119,
351-367.
-
Moreira, M., K. Breddam, J.
Curtice and M.D. Kurz, (2001). Solar neon in the
Icelandic mantle: new evidence for an undegassed
lower mantle, Earth Planet. Sci. Lett.
185, 15-23.
-
Natland, J.H., (2003). Capture
of mantle helium by growing olivine phenocrysts
in picritic basalts from the Juan Fernandez Islands,
SE Pacific, J. Pet. 44,
421-456.
-
Nier, A. O. and Schlutter, D.
J. (1990). Helium and neon in stratospheric particles.
Meteoritics 25, 263-267.
-
O’Nions, R. K. and Oxburgh,
E. R. (1983). Heat and helium in the Earth. Nature
306, 429-436.
-
Ozima, M. and Zashu, S. (1988).
Solar-type Ne in Zaire cubic diamonds. Geochim.
Cosmochim. Acta 52, 19-25.
-
Ozima, M. and Zashu, S. (1991).
Noble gas state of the ancient mantle as deduced
from noble gases in coated diamonds. Earth Planet.
Sci. Lett. 105, 13-27.
-
Ozima M. 1994. Noble gas state
in the mantle. Rev. Geophys. 32,
405-426.
-
Ozima M, Wieler R, Marty B, Podosek
F (1998). Comparative studies of solar, Q-gases,
and terrestrial noble gases, and implications on
the evolution of the solar nebula. Geochimica
et Cosmochimica Acta, 62,
301-314
-
Ozima, M. and G. Igarashi, (2000).
The primordial noble gases in the Earth: a key constraint
on Earth evolution models, Earth Planet. Sci.
Lett. 176, 219-232.
-
Ozima, M. & F.Podosek, (2002).
Noble Gas Geochemistry, Cambridge University
Press, New York.
-
Sarda, P. and D.W. Graham, (1990).
Mid-ocean ridge popping rocks: implications for
degassing at ridge crests, Earth Planet. Sci.
Lett. 97, 268-289.
-
Seta, A., Matsumoto, T. and Matsuda,
J.-I. (2001). Concurrent evolution of 3He/4He
ratio in the Earth’s mantle reservoirs for
the first 2 Ga. Earth Planet. Sci. Lett.
188, 211–9.
-
Staudacher, T., Sarda, P., Richardson,
S. H., Allegre, C. J., Sagna, I. and Dmitriev, L.
V. (1989). Noble gases in basalt glasses from a
Mid-Atlantic Ridge topographic high at 14°N:
geodynamic consequences. Earth Planet. Sci.
Lett. 96, 119-133.
-
Stuart, F.M., S. Lass-Evans,
J.G. Fitton and R.M. Ellam, (2003). Extreme 3He/4He
in picritic basalts from Baffin Island: the role
of a mixed reservoir in mantle plumes, Nature
424, 57-59.
-
van Keken, P. E. and Ballentine,
C. J. (1998). Whole-mantle versus layered mantle
convection and the role of a high-viscosity lower
mantle in terrestrial volatile evolution. Earth
Planet. Sci. Lett. 156, 19–32.
-
van Keken, P. E. and Ballentine,
C. J. (1999). Dynamical models of mantle volatile
evolution and the role of phase transitions and
temperature-dependent rheology. J. Geophys.
Res. 104, 7137–51.
|
last updated 17th
June, 2004 |
|
|
